
JUVÉDERM VOLLURE™ XC Approved by U.S. FDA for Correction of Facial Wrinkles and Folds
Allergan, a leading global pharmaceutical company that also manufactures Botox and Kybella, recently announced that the company has received approval from the U.S. Food and Drug Administration (FDA) to market Juvederm Vollure XC for the correction of facial wrinkles and folds.
“The FDA approval of JUVÉDERM VOLLURE™ XC demonstrates Allergan’s imperative to develop next-generation HA fillers designed to meet different patient needs,” said David Nicholson, Chief Research and Development Officer at Allergan.
“This commitment to ongoing scientific research and development is one of the factors that make JUVÉDERM®, the number one selling collection of dermal filler products.”
Vollure joins the Juvederm family of fillers which also includes popular HA products such as JUVÉDERM VOLUMA® XC, JUVÉDERM® XC, JUVÉDERM® Ultra XC and JUVÉDERM VOLBELLA®.
Vollure is formulated with Allergan’s proprietary VYCROSS® technology, which according to Allergan blends different molecular weights of hyaluronic acid, contributing to the gel’s duration.
VYCROSS® technology is also featured in JUVÉDERM VOLUMA® XC, which was FDA-approved to increase volume lost due to aging in the cheek area.
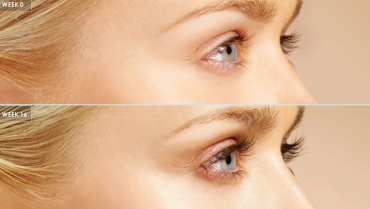
Now with JUVÉDERM VOLLURE™ XC, the advanced VYCROSS® technology yields a custom engineered injectable gel product which was studied in the nasolabial folds, the number one dermal treatment area.
Vollure delivers a long-lasting result, up to 18 months.
According to the Vollure clinical trials, 82 percent of patients said they were very satisfied after six months and 68 percent still claimed satisfaction at 18 months.
Vollure is the first and only Hyaluronic Acid (HA) Dermal Filler that is FDA approved for the correction of moderate to severe wrinkles and folds, such as nasolabial folds.
While Vollure will be brand new in the United States, the filler has been approved in Europe since 2013 under the name Juvéderm Volift and has experienced success in more than 80 countries outside of the U.S. since then.
JUVÉDERM VOLLURE™ XC is expected to be available in offices starting in April of 2017.


Add Comment