JUVÉDERM VOLBELLA® XC Approved By U.S. FDA For Use In Lips And Perioral Rhytids
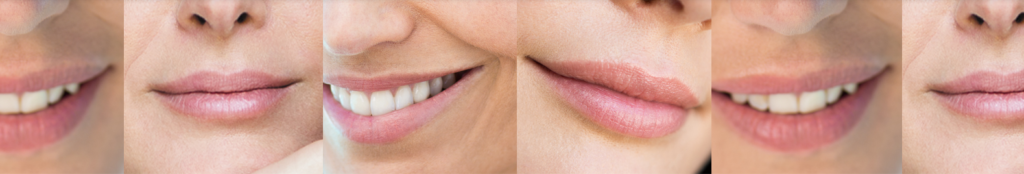
JUVÉDERM VOLBELLA® XC found to increase lip fullness and soften the appearance of lines around the mouth through one year.
DUBLIN, June 1, 2016 /PRNewswire/ — Allergan plc, (NYSE: AGN), a leading global pharmaceutical company, today announced that the company has received approval from the U.S. Food and Drug Administration (FDA) to market JUVÉDERM VOLBELLA® XC, for use in the lips for lip augmentation and for correction of perioral rhytids, commonly referred to as perioral lines, in adults over the age of 21.1 In clinical trials, JUVÉDERM VOLBELLA® XC was found to effectively increase lip fullness and soften the appearance of lines around the mouth in a majority of subjects through one year.1,2*
“Many of my patients are very bothered by the lines that can appear around the lips, known as perioral rhytids. Additionally, when seeking lip augmentation, patients want a smooth, result that is not drastic” said Dr. David E. Bank, clinical trial investigator and founder of The Center for Dermatology, Cosmetic & Laser Surgery. “JUVÉDERM VOLBELLA® XC adds fullness to the lips and softens the appearance of the lines around the lips.2 With JUVÉDERM VOLBELLA® XC I am able to subtly enhance my patients’ pout.”
“The FDA approval of JUVÉDERM VOLBELLA® XC further demonstrates Allergan’s commitment to developing advanced products and technologies that allow healthcare providers to better address evolving patient needs,” said Bill Meury, Chief Commercial Officer, Allergan.
“Additionally, this approval brings to market a product unlike anything that is currently available in the United States. JUVÉDERM VOLBELLA® XC is formulated with VYCROSS®,2 a proprietary filler technology from Allergan, which yields smooth products that have been engineered to address specific patient concerns such as lip fullness, age-related volume loss in the cheek area, or perioral rhytids.3”
VYCROSS® blends different molecular weights of hyaluronic acid which contributes to the gel’s duration.1,3 In addition, JUVÉDERM VOLBELLA® XC has been customized with a lower HA concentration (15 mg/mL), while still providing the long-lasting results healthcare providers expect from the JUVÉDERM® collection of fillers.1,4,5,6,7,8 This makes JUVÉDERM VOLBELLA® XC a soft, smooth gel appropriate for adding subtle volume to the lips and softening the appearance of perioral lines.1
Allergan first debuted this innovative VYCROSS® technology in the U.S. in 2013 with the FDA approval of JUVÉDERM VOLUMA® XC for age-related mid-face volume loss.4 Now JUVÉDERM VOLBELLA® XC is the latest addition to the JUVÉDERM® collection of fillers, the number one selling collection of dermal filler products in the world,9 to receive FDA approval.
The safety and effectiveness of JUVÉDERM VOLBELLA® XC has been demonstrated in several clinical trials including the U.S. pivotal study where 168 subjects were treated with JUVÉDERM VOLBELLA® XC. A 5-point scale was used to evaluate the effectiveness of the product for lip fullness and a 4-point scale to evaluate the effectiveness of the product for smoothing lines around the mouth.2
Approximately two-thirds of subjects treated with VOLBELLA XC showed improvement in lip fullness and perioral lines through 1 year. The safety of JUVÉDERM VOLBELLA® XC was observed to be similar to that of the control. The most common side effects were temporary responses at the treatment site such as swelling, tenderness, bruising, firmness lumps/bumps, rednesss, pain, discoloration, and itching. Most of these side effects resolved within 30 days.2
JUVÉDERM VOLBELLA® XC was first approved in Europe in 2011. Currently, JUVÉDERM VOLBELLA® XC is distributed in more than 70 countries, including markets in Europe, Latin America, Middle East, Asia Pacific, and Canada. The JUVÉDERM® family of products, including JUVÉDERM® Ultra XC and JUVÉDERM® Ultra Plus XC are marketed and sold in more than 80 countries outside the United States.10
JUVÉDERM VOLBELLA® XC will be available to patients in October 2016. For more information about JUVÉDERM VOLBELLA® XC and the JUVÉDERM® collection of fillers or to find a doctor, please visit www.juvederm.com.



Add Comment